Sport
Dollar
38,2552
0.34 %Euro
43,8333
0.15 %Gram Gold
4.076,2000
0.31 %Quarter Gold
6.772,5700
0.78 %Silver
39,9100
0.36 %Africa Statistics Day is observed on November 18 to highlight the importance of data and statisticians to development.
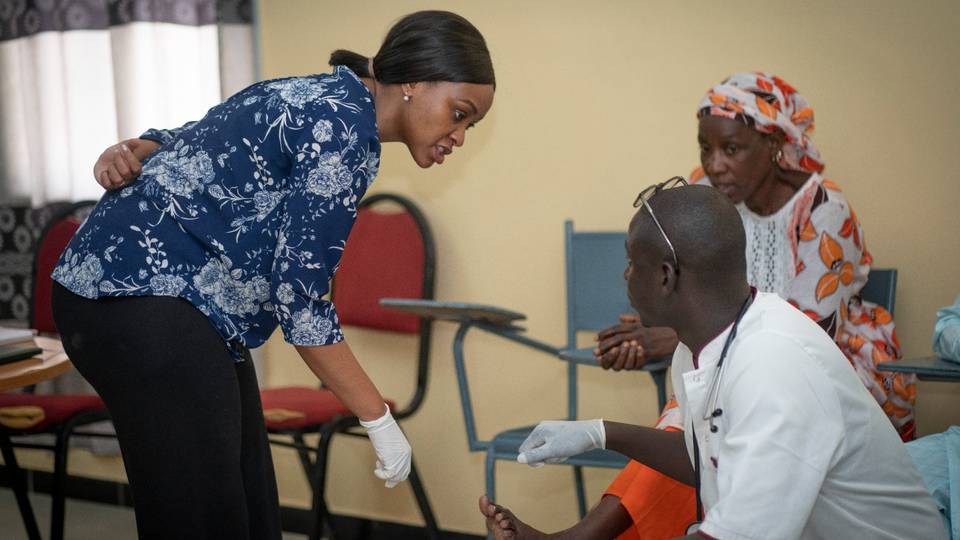
By Collins Mutua
Have you ever wondered how the pills in your medicine cabinet actually make it to you?
It takes a village to develop new medicines. The village comprises researchers, clinicians, clinical trial participants, regulatory authorities, and distributors.
Drug companies undergo a rigorous process of experimentation to discover new drugs and assess drugs’ safety in labs before conducting clinical trials in humans.
Once safety and efficacy are determined in clinical trials, regulatory agencies review the findings and approve new drugs for production and marketing.
Drugs then undergo post-marketing monitoring to ensure their continued safety and efficacy.
However, the unsung heroes of the drug development process are often data managers and statisticians.
Neglected diseases
For instance, in 2003, a team of scientists from Sudan, Uganda, Kenya, and Ethiopia collaborated to find a better treatment for the fatal parasitic disease known as visceral leishmaniasis, or kala-azar.
The existing treatment at the time was a 30-day course of daily painful injections that were expensive and had frequent side effects.
A highly skilled group of statisticians, data managers, and programmers who provided centralised clinical data management supported the scientists.
The statisticians developed the trial protocols to ensure that the study was designed to prove scientifically that the new medicine has the intended effect.
The data managers developed the clinical database, ensuring that the data collected during the trial was complete, clean, and error-free when transferred and recorded into the database.
The statistical programmers then prepared the data for analysis, after which the statisticians stepped in again to determine the safety and efficacy of the drug, interpret the results, and write the study report.
Better treatment
Seven years later, in March 2010, the World Health Organization (WHO) recommended this new treatment, which is safer, more effective, and only takes 17 days for patients to complete.
It had a significant impact on the lives of thousands of neglected kala-azar patients in Eastern Africa, half of whom were children and most of whom came from hard-to-reach and impoverished communities.
Thanks to these statisticians’ collective efforts, a new and better treatment was developed for this neglected disease.
Neglected tropical diseases (NTDs) affect 1.6 billion people every year, and Africa carries almost 40% of the worldwide burden. In my home country of Kenya, more than 25 million people are affected by 15 of the 20 WHO-listed NTDs.
Unfortunately, for too many of them, existing treatments are not good enough, some have a low cure rate or severe side effects that can even cause death.
These patients are often overlooked by traditional pharmaceutical research because they are too poor to constitute a lucrative market.

The challenges
I’m proud to be part of the team of statisticians that contributed to developing a new and better treatment for kala-azar in Eastern Africa.
Since our founding in 2004 by the non-profit medical research organization Drugs for Neglected Diseases initiative (DNDi), we have supported over a dozen clinical trials for neglected diseases.
The only operation of its kind in Africa, we work not only with DNDi but also with other organizations, including the WHO, Médecins Sans Frontières (MSF), and the Kenya Medical Research Institute (KEMRI).
Our support for clinical trials for other diseases, such as multidrug-resistant tuberculosis and Buruli ulcer, has significantly contributed to the development of better treatments.
Recently, we conducted a study in Sudan on a new treatment for mycetoma, a chronic, disabling disease that leads to severe deformities. We are hopeful that our study's results will lead to better medicines for people suffering from this horrific disease.
Robust studies
However, working on neglected diseases comes with its own set of challenges.
First, due to the lack of attention these diseases receive, there is often insufficient data to determine the appropriate number of participants for a trial.
This number is typically based on existing research, which is often scarce for neglected diseases. In such cases, alternative methods such as pilot studies or simulations may be required, which are time-consuming and costly.
Researchers simply need more investments in clinical research of neglected diseases as this will lead to new treatment options for people who are vulnerable to these diseases.
Trials on new health interventions are likely to produce the clearest results when carried out in diverse settings.
That’s why we also need more clinical trial data and statistical hubs locally to avoid shipping lab samples abroad and experiencing long delays in receiving results.
Secondly, the lack of physical infrastructure, such as roads and internet connectivity, still makes it difficult to prove the viability of clinical research in remote settings.
To overcome part of this challenge, we have adopted electronic data capture systems that come with offline modules, which enables us to collect data directly from the site into the clinical database without internet.
African governments should, therefore, prioritise infrastructure development and facilitate the creation of regional platforms that enable stronger South-South collaborations.
Lastly, some countries have strict data regulations and laws. While it is vital to respect data protection laws, it is also essential to find ways to combine anonymised patient data from many different countries to pool analysis, obtain statistical power sufficient for robust studies, and make this data available to researchers across borders.
Africa and the rest of the world must take pride in the efforts of medical statisticians, especially in their contribution to the fight against neglected diseases.
Their continuous provision of evidence-based clinical research results has been instrumental in providing the best science for the most neglected.
Collins Mutua is the Head of Biostatistics at the Drugs for Neglected Diseases initiative (DNDi)
Disclaimer: The views expressed by the author do not necessarily reflect the opinions, viewpoints and editorial policies of TRT Afrika.
Comments
No comments Yet



















Comment